Anavex Life Sciences Reports Fiscal 2021 Second Quarter Financial Results And Business Outlook
- ANAVEX® Life Sciences Corp.
- May 13, 2021
- 4 min read
Updated: Sep 22, 2021
Conference Call and Webcast Today at 4:30 p.m. ET

NEW YORK – May 13, 2021
Anavex Life Sciences Corp. (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company developing differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders including Alzheimer’s disease, Parkinson’s disease, Rett syndrome and other central nervous system (CNS) diseases, today reported financial results for its fiscal quarter ended March 31, 2021.
“Positive momentum continues to build this quarter and affirms the strength of our pipeline and execution capabilities,” said Christopher U Missling, PhD, President and Chief Executive Officer of Anavex. “For our lead drug candidate, ANAVEX®2-73 in Alzheimer’s disease, we are close to complete enrollment for our Phase 2b/3 study, and we expect a very data-rich current quarter with data readouts from multiple clinical trials and the remainder of 2021 to be a catalyst-rich year for Anavex. This is an exciting time for Anavex and I look forward to advancing our impressive pipeline of mid- and late-stage clinical trials with a commitment to bringing innovative therapies to patients in need around the globe.”
Anavex Life Sciences’ Business and Near-Term Clinical Outlook:
ANAVEX®2-73 Program and other Near-Term Pipeline Data Updates:
Complete data ANAVEX®2-73 U.S. Rett syndrome Phase 2 study.
Complete data ANAVEX®2-73 Parkinson’s disease dementia (PDD) Phase 2 study.
Top-line data AVATAR: Potentially pivotal Phase 2/3 ANAVEX®2-73 adult Rett syndrome clinical trial.
Top-line data Phase 1 ANAVEX®3-71 clinical trial.
Complete enrollment of Phase 2b/3 ANAVEX®2-73 Alzheimer’s disease clinical trial.
Recent Business Highlights:
In March 2021, Anavex announced that the Independent Data Safety Monitoring Board (DSMB) for the Company’s Phase 2b/3 Alzheimer’s disease study of its investigational compound ANAVEX®2-73 (blarcamesine) has completed its recent pre-planned review of the preliminary Phase 2b/3 study data. As specified in the protocol, the DSMB reviewed the interim safety data for the ANAVEX®2-73 Phase 2b/3 Alzheimer’s disease clinical study ANAVEX®2-73-AD-004 and its Open Label Extension (OLE) ANAVEX®2-73-AD-EP-004 ATTENTION-AD study. Upon review of the interim safety data, the DSMB made the following recommendation: The DSMB recommendation is to continue the studies without modification.
In April 2021, Anavex announced that the Independent Data Safety Monitoring Board (DSMB) for the Company’s ongoing clinical trial program, including the late-stage AVATAR (ANAVEX®2-73-RS-002), EXCELLENCE (ANAVEX®2-73-RS-003) and the U.S. Rett syndrome extension study (ANAVEX®2-73-RS-EP-001) of its investigational compound ANAVEX®2-73 (blarcamesine) has completed its recent pre-planned review of the respective interim safety data for these three separate clinical studies. Upon review of the interim safety data, the DSMB made the following recommendation for each of the three studies: The DSMB recommendation is to continue the studies without modification.
In March 2021, Anavex announced that ANAVEX®2-73 (blarcamesine) is featured in a recent peer-reviewed publication in the journal of Neuropharmacology, titled “Future avenues for Alzheimer’s disease detection and therapy: liquid biopsy, intracellular signaling modulation, systems pharmacology drug discovery” from the series of the special issue on ‘The Quest for Disease-Modifying Therapies for Neurodegenerative Disorders’.
In May 2021, Anavex announced the appointment of former FDA officer as Senior Vice President for Nonclinical Development.
Further clinical milestones are provided in Anavex Life Sciences’ latest corporate presentation, available on anavex.com.
Financial Highlights:
Continued fiscal responsible management of cash utilization.
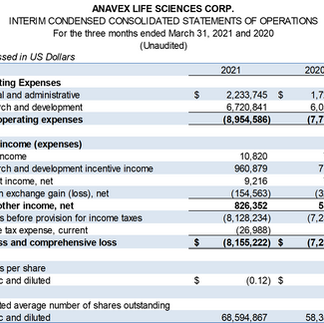
Cash and cash equivalents at March 31, 2021 of approximately $75.9 million, sufficient cash runway for up to three (3) years.
Net loss of $8.2 million, or $0.12 per share for the quarter, compared to net loss of $7.2 million, or $0.12 per share in comparative quarter of fiscal 2020.
Research and development expenses of $6.7 million for the quarter, compared to $6.1 million for comparable quarter of fiscal 2020.
General and administrative expenses were $2.2 million for the quarter, as compared to $1.7 million for the prior year period.
The financial information for the fiscal quarter ended March 31, 2021 should be read in conjunction with the Company’s interim condensed consolidated financial statements, which will appear on EDGAR, www.sec.gov and will be available on the Anavex website at www.anavex.com.
Conference Call / Webcast Information:
The live webcast of the conference call can be accessed online at https://wsw.com/webcast/cc/avxl18/1499544.
To join the conference call, live via telephone, interested parties within the U.S. should dial, toll-free, 1 (866) 866-1333 and international callers should dial 1 (404) 260-1421. Please use confirmation number 50162864, followed by the pound sign (#).
A replay of the conference call will also be available on www.anavex.com.
About Anavex Life Sciences Corp.
Anavex Life Sciences Corp. (Nasdaq: AVXL) is a publicly traded biopharmaceutical company dedicated to the development of differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental disorders including Alzheimer’s disease, Parkinson’s disease, Rett syndrome and other central nervous system (CNS) diseases, pain and various types of cancer. Anavex’s lead drug candidate, ANAVEX®2-73 (blarcamesine), successfully completed a Phase 2a clinical trial for Alzheimer’s disease and recently a Phase 2 proof-of-concept study in Parkinson’s disease dementia and a Phase 2 study in adult patients with Rett syndrome. ANAVEX®2-73 is an orally available drug candidate that restores cellular homeostasis by targeting sigma-1 and muscarinic receptors. Preclinical studies demonstrated its potential to halt and/or reverse the course of Alzheimer’s disease. ANAVEX®2-73 also exhibited anticonvulsant, anti-amnesic, neuroprotective and anti-depressant properties in animal models, indicating its potential to treat additional CNS disorders, including epilepsy. The Michael J. Fox Foundation for Parkinson’s Research previously awarded Anavex a research grant, which fully funded a preclinical study to develop ANAVEX®2-73 for the treatment of Parkinson’s disease. ANAVEX®3-71, which targets sigma-1 and muscarinic receptors, is a promising clinical stage drug candidate demonstrating disease-modifying activity against the major hallmarks of Alzheimer’s disease in transgenic (3xTg-AD) mice, including cognitive deficits, amyloid and tau pathologies. In preclinical trials, ANAVEX®3-71 has shown beneficial effects on mitochondrial dysfunction and neuroinflammation. Further information is available at www.anavex.com. You can also connect with the company on Twitter, Facebook and LinkedIn.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks set forth in the Company’s most recent Annual Report on Form 10-K filed with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement and Anavex Life Sciences Corp. undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof.
For Further Information:
Anavex Life Sciences Corp. Research & Business Development Toll-free: 1-844-689-3939 Email: info@anavex.com
Investors:
Andrew J. Barwicki Investor Relations Tel: 516-662-9461 Email: andrew@barwicki.com